|
|
|
|
1. Revealing telomere's mysterious by chemical approaches
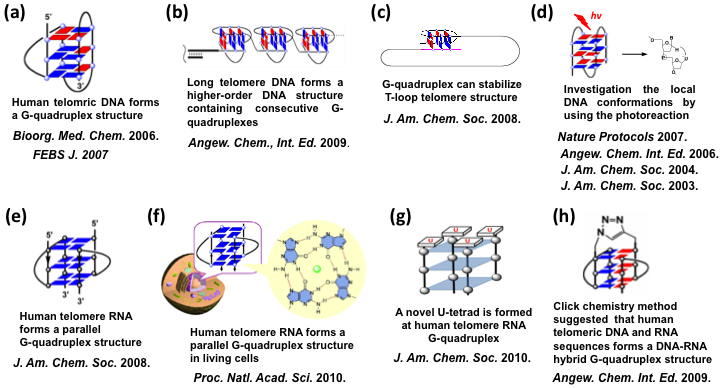
Using artificial nucleic acids, we found that human telomric DNA formed a G-quadruplex structure (News story describing this work was highlighted in Chem. & Eng. News, 2006, July 31, American Chemical Society) (Fig. a). Furthermore, we found that long telomere DNA forms a higher-order DNA structure containing consecutive G-quadruplexes by using atomic force microscopy. (Highlighted on Nature Asia as the top one most viewed research highlights, November 16 2009) (Fig. b). Our recent studies also demonstrated that a G-quadruplex can stabilize T-loop telomere structure (Fig. c). We have investigated the local DNA conformations by using the photoreactions of 5-halouracil-containing DNA. It was suggested that the product of 2'-deoxyribonolactone residue is effectively produced only in the antiparallel G-quadruplex (Fig. d).
We investigated the structural features of human telomere RNA. The NMR showed that human telomere RNA forms a parallel G-quadruplex structure (Fig. e). Furthermore, using a light-switching probe, we found that human telomere RNA forms a parallel G-quadruplex structure in living cells, providing the in vivo evidence for the presence of the G-quadruplex in human (Fig. f). We also demonstrated that a novel U-tetrad is formed at human telomere RNA G-quadruplex (Fig. g). The unique structural feature may provide new targets for efforts directed toward the design of potent and selective RNA G-quadruplex-interacting molecules. These results provide valuable information to allow understanding of the structure and function of human telomere RNA.
Recently, we developed a simple "click chemistry" method to detect G-quadruplex structure. By using this approach we discovered a DNA-RNA hybrid-type G-quadruplex structure formed from human telomeric DNA and RNA sequences (Fig. h). Furthermore, we demonstrated that formation of such an intermolecular G-quadruplex by telomeric RNA and DNA contributes to telomere end protection.
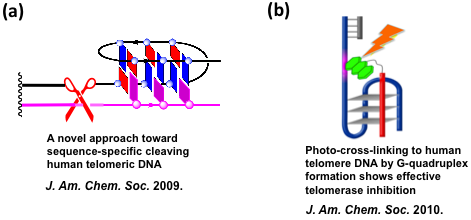
2. Developing various chemical approaches to targeting human telomeres and
telomerase for the treatment of cancer
Recently, we developed a novel approach toward sequence-specific cleaving human telomeric DNA. EDTP as metal binding group was conjugated to an oligonucleotide DNA. Oligonucleotide probe DNA bearing this kind of metal-affinity group were efficiently recruiting the catalytic species to the target site by G-quadruplex formation and resulting in an efficient cleavage at telomere DNA (Fig. a). To further expand this research, we developed a small 6-mer oligonucleotide with a photo-cross-linking reagent to cause efficient photo-cross-linking to human telomere DNA upon light irradiation. Because the main effect on the target is controllable by switching the light on/off, the small, photocontrolled oligonucleotide can minimize side effects by irradiation of only cancer cells, which alleviates the main problem with present-day anticancer agents. (Fig. b).